Biological Science Faculty Member - Retired
 Dr. Kenneth H. Roux
Dr. Kenneth H. Roux
| Office: | (850) 644-5037 |
| Fax: | (850) 644-0481 |
| Mail code: | 4370 |
| E-mail: |
roux@bio.fsu.edu |
Professor;
Ph.D., Tulane University, 1974
Graduate faculty status
Research and Professional Interests:
We have three major projects in the lab at this time:
- Food Allergen Research
- AIDS Structural Research
- Structural Analyses of Immunoglobulins and Immune Complexes
1. Food Allergen Research:
About 3% of the population has some sort of allergic response to one or more foods and 0.5% are specifically allergic to tree nuts. Other patients have IgE antibodies to nut proteins but dont show allergic symptoms. We have been identifying allergies in cashew, walnut, almond and pistachio using serum from allergic patients to screen cDNA expression libraries. Once cloned and expressed, the offending proteins are subjected to epitope mapping techniques and mutagenesis to generate hypoallergenic version. At the same time, we are developing polyclonal and monoclonal antibodies to tree nut allergens to be used by the food industry in testing suspected foods for contamination with allergens.
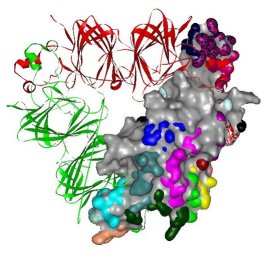
Model of cashew 11S globulin, a potent trimeric allergen, in which the IgE-binding sites (colored residues) are highlighted on one subunit (grey).
2. AIDS Structural Research:
Electron microscopic analysis of AIDS virus envelope (Env) proteins. The Env spikes on the surface of HIV-1 and related viruses foster viral fusion with target T-helpers cells and macrophages and are the targets for neutralizing antibodies. We have analyzed several molecular forms that are being considered as vaccine candidates. We are particularly interested in using well characterized neutralizing antibodies to map the surface of gp120 and its precursor molecule, gp160. Extensive use is made of negative stain electron microscopic for these analyses.
|
 |
|||
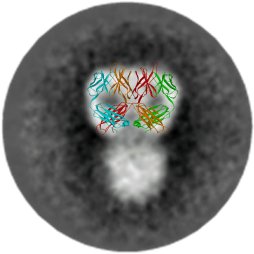
| AIDS virus (SIV) envelope spike model derived by cryoelectron microscopy tomography of over 6,000 spikes. Zhu, et al. Nature 441: 847-852, 2006. | Averaged electron microscopic image and X-ray model of the HIV-1 neutralizing antibody, 2G12, showing a unique domain-exchanged structure of the heavy chains such that the Fab arms are locked together. Calarese et al., Science 300: 20652071, 2003. |
Tomographic analysis of HIV-1 and SIV. We have recently published the first detailed look at the surface features of the AIDS viruses by using negative stain and cryoelectron microscopic tomography techniques to generate 3-D images of viruses. The envelope spikes were found to be few in number and somewhat clustered on the surface of HIV-1. By averaging the spike images on SIV, we generated a 3-D image of the spike and tentatively fitted the know crystal structures of the gp120 and gp41 subunits within the spike density. We are particularly interested in viewing the binding of neutralizing antibodies and CD4 in complex with the envelope spikes on the surface of virions.
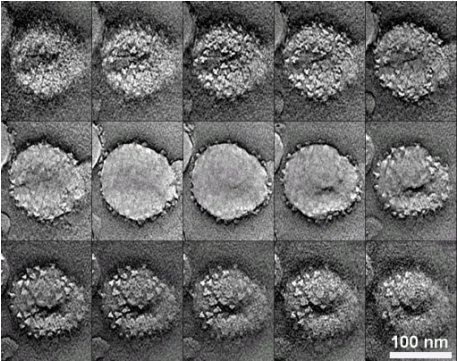
3-D tomogram (serial layers) of a single SIV virus particle showing surface envelope spikes. Zhu et al., Proc. Natl. Acad. Sci. USA, 100: 15812-15817, 2003.
3. Structural Analyses of Immunoglobulins and Immune Complexes:
We have used negative stain electron microscopy to study a wide variety of immunoglobulins and immune complexes with a focus on immunoglobulin structure and flexibility, antigen structure and ligand interaction, and epitope mapping.
.jpg) |
| Soluble HIV-1 gp120 trimer (central mass) with three soluble four-domain CD4 ligands (radial arms) and fitted model. Kang et al. Vaccine, 27: 5120-5132. 2009. |
Selected Publications:
Allergy Publications
Mandalari, G., Rigby, N. M., Bisignano, C., Lo CurtoR. B., Mulholland, F., Su, M., Venkatachalam, M., Robotham, J. M., Willison, L. N., Lapsley, K., Roux, K. H., Sathe, S. K. Effect of food matrix and processing on release of almond protein during simulated digestion. LWT - Food Research International. (in press)
Gieras, A., Linhart, B., Roux, K. H., Dutta, M., Khodoun, M., Zafred, D., Cabauatan, C. R., Lupinek, C., Weber, M., Focke-Tejkl, M., Keller, W., Finkelman, F. D., Valenta, R. IgE epitope proximity determines immune complex shape and effector cell activation capacity. J. Allergy Clin. Immunol. (in press)
Su, M., Venkatachalam, M., Gradziel, T. M., Liu, C., Zhang, Y., Roux, K. H., and Sathe, S. K. Application of mouse monoclonal antibody (mAb) 4C10-based enzyme-linked immunosorbant assay (ELISA) for amandin detection in almond (Prunus dulcis L.) genotypes and hybrids. LWT-Life Science and Technology (in press) 60:535-543. 2015. http://dx.doi.org/10.1016/j.lwt.2014.08.042
Guan, X, Noble, K. A., Tao, Y., Roux, K. H., Sathe, S. K., Young, N. L., Marshall, A. G. Epitope mapping of 7S cashew antigen in complex with antibody by solution-phase H/D exchange monitored by FT-ICR mass spectrometry. J. Mass, Spectrom. 50:812-819. 2015. DOI 10.1002/jms.3589.
Willison, L. N., Sathe, S. K., Roux, K. H. Production and analysis of recombinant tree nut allergens. Methods, 66:34-43. 2014. http://dx.doi.org/10.1016/j.ymeth.2013.07.033.
Dhakal, S., Liu, C., Zhang,Y., Roux, K. H., Sathe, S. K., Balasubramaniam, V. M. Effect of high pressure processing on the immunoreactivity of almond milk. Food Research International 62:215-222. 2014. http://dx.doi.org/10.1016/j.foodres.2014.02.021
Willison, L. N., Zhang, Q., Su, M., Teuber, S. S., Sathe, S. K., and Roux, K. H. Conformational epitope mapping of Pru du 6, a major allergen from almond nut. Mol. Immunol. 55: 253-263. 2013. http://dx.doi.org/10.1016/j.molimm.2013.02.004.
Sharma, G., Irsigler, A., Dhanarajan, P., Ayuso, R., Bardina, L., Sampson, H. A., Roux, K. H., Sathe, S. K. Cloning and characterization of 2S albumin albumin, Car i 1, a major allergen in pecan. J. Agric. Food Chem. 59:4130-4139. 2011.
Kshirsagar, H. H.; Fajer, P.; Sharma, G. M.; Roux, K. H., Sathe, S. K. Biochemical and spectroscopic characterization of almond and cashew nut seed 11S legumins, amandin and anacardein. J. Agric. Food Chem. 59:386-393. 2011.
Willison, L. N., Tripathi, P., Sharma, G., Teuber, S. S., Sathe, S. K. and Roux, K. H. Cloning, expression, and patient IgE reactivity of recombinant Pru du 6, an 11S globulin from almond. Int. Arch. Allergy Immunol. 156:267-281. 2011. DOI: 10.1159/000323887.
Sharma, G. M., Mundoma, C., Seavy, M., Roux, K. H., and Sathe, K. Purification and biochemical characterization of Brazil nut (Bertholletia excels L.) seed storage proteins. J. Agric. Food Chem. 58:5714-5723. 2010.
Xia, L., Willison, L. N., Porter, L., Robotham, J. M., Teuber, S. S., Sathe, S. K., and Roux, K. H. Mapping of a conformational epitope on the cashew allergen Ana o 2: A discontinuous large subunit epitope dependent upon homologous or heterologous small subunit association. Mol. Immunol. 47:1808-1816, 2010
[PDF]
Robotham, J. M., Xia, L., Willison, L. N., Teuber, S. S., Sathe, S. K., and Roux, K. H. Characterization of a cashew allergen, 11S globulin (Ana o 2), conformational epitope. Mol. Immunol. 47:1830-1838, 2010
[PDF]
Robotham, J. M., Hoffman, G. G., Teuber, S. S., Beyer, K., Sampson, H. A., Sathe, S. K., and Roux, K. H. Linear IgE-epitope mapping and comparative structural homology modeling of hazelnut and English walnut 11S globulins. Mol. Immunol. 46:2975-2984. 2009.
[PDF]
Sathe, S. K., Sharma, G. M., Kshirsagar, H. H., Su, M., and Roux, K. H. Effects of long term storage on electrophoretic patterns, immunoreactivity, and in vitro digestibility of soybean (Glycine max L.) proteins J. Agric. Food Chem. 57:769-776. 2009
[PDF]
Sharma, G. M., Roux, K. H and Sathe, S. K. A sensitive and robust competitive enzyme-linked immunosorbent assay for Brazil nut (Bertholletia excelsa L.) detection. J. Agric. Food Chem. 57:769-776. 2009.
[PDF]
Venkatachalam, M., Monaghan, E. K., Kshirsagar, H. H., Robotham, J. M., ODonnell, S. E., Gerber, M. S., Roux, K. H., and Sathe, S. K. Effects of processing on antigenic stability of cashew nut (Anacardium occidentale L.) proteins. J. Agric. Food Chem. 56:8998-9005. 2008.
[PDF]
Willison, L. N., Tawde, P., Robotham, J. M. Penney, R., Teuber, S. S., Sathe, S. K., Roux, K. H. Pistachio vicilin, Pis v 3, is IgE-reactive and cross-reacts with the homologous cashew allergen, Ana o 1. Clin. Exp. Allergy 38:1220-1238. 2008.
[PDF]
Tawde, P., Venkatesh, Y. P., Wang, F., Teuber, S. S., Sathe, S, K., and Roux, K. H. Cloning and characterization of profilin (Pru du 4), a cross-reactive almond (Prunus dulcis) allergen. J. Allergy Clin. Immunol. 118:915-922. 2006.
[PDF]
Robotham, J. M., Wang, F., Seamon, V, Teuber, S. S., Sathe, S. K., Beyer, K, Sampson, H. and Roux, K. H. Ana o 3, an important cashew nut (Anacardium occidentale L.) allergen of the 2S albumin family. J. Allergy Clin. Immunol. 15:1284-1290, 2005.
[PDF]
Sathe, S. K., Teuber, S. S., Roux, K. H. Effects of food processing on the stability of food allergens. Biotech. Adv. 232:423-429, 2005.
[PDF]
Roux, K. H., S. S. Teuber, and S. K. Sathe. Tree nut allergens. Int. Arch. Allergy Immunol., 131:234-244. 2003.
[PDF]
Wang, F., J. M. Robotham, S. S. Teuber, S. K. Sathe, and K. H. Roux. Ana o 2, a major cashew nut (Anacardium occidentale L.) allergen of the legumin family. Int. Arch. Allergy Immunol., 132:27-39. 2003.
[PDF]
Wei, Y., K. Shridhar, S. K. Sathe, S. S. Teuber, and. K. H. Roux. A sensitive sandwich ELISA for the detection of trace amounts of cashew (Anacardium occidentale) nut in foods. J. Agric. Food Chem. 51:3215-3221. 2003.
[PDF]
Robotham, J. M., Teuber, S. S., Sathe, S. K., and Roux, K. H. Linear IgE epitope mapping of the English walnut (Juglans regia) major food allergen, Jug r 1. J. Allergy Clin. Immunol. 109:143-149, 2002.
[PDF]
Wang, F., J. M. Robotham, S. S. Teuber, P. Tawde, S. K. Sathe, and K. H. Roux. Ana o 1, a cashew (Anacardium occidentale) allergen of the vicilin seed storage protein family. J. Allergy Clin. Immunol. 110:160-166. 2002.
[PDF]
AIDS Publications
Dutta, M., Liu, J., Roux, K. H., and Taylor, K. A. Visualization of retroviral envelope spikes in complex with the V3 loop antibody 447-52D on intact viruses by cryo-electron tomography. J. Virol. 88:12265-75. 2014.
Rathinakumar, R., Dutta, M., Zhu, P., Johnson, W., and Roux, K. H. Binding of anti-membrane proximal gp41 monoclonal antibodies to CD4-liganded and unliganded human immunodeficiency virus Type 1 and Simian immunodeficiency virus virions. J. Virol. 86:1820-31. 2012.
Hu, G., Liu, J., Taylor, K. A., and Roux, K. H. Structural comparison of HIV-1 envelope spikes with and without the V1/V2 loop. J. Virol. 85:2741-2750. 2011.
Kang, Y, Andjelic, S, Binley, J. M., Crooks, E. T., Franti, M., Iyer S. P. N., Donovan, G. P., Dey, A. K., Zhu, P., Roux, K. H., Durso, R. J., Parsons, T., Maddon, P. J., Moore, J. P. and Olson, W. C. Structure and immunogenicity studies of a cleaved, stabilized envelope trimer derived from subtype A HIV-1. Vaccine, 27:5120-5132. 2009. doi:10.1016/j.vaccine.2009.06.037
[PDF]
Zhu, P. Winkler, H., Chertova, E., Taylor, K. A., and Roux, K. H. Cryoelectron tomography of HIV-1 envelope spikes: further evidence for tripod-like legs. PLoS Pathogens 4: doi:10.1371/journal.ppat.1000203. 2008.
[PDF]
Selvarajah, S., Puffer, B. A., Lee, F.-H. Zhu, P., Li, Y.,Wyatt, R, Roux, K. H., Doms, R. W., and Burton, D. R. Focused dampening of antibody responses to the immunodominant variable loop by engineered soluble gp140. AIDS Res. Human Retroviruses. 24:301-314. 2008.
[PDF]
Crooks, E. T, Moore, P. L., Franti, M., Cayanan, C. S., Zhu, P., Jiang, P., de Vries, R. P., Wiley, C., Zharkikh, I, Schülke, N., Roux, K. H., Montefiori, D. C., Burton, D. R, and Binley, J. M. A comparative immunogenicity study of HIV-1 virus-like particles bearing various forms of envelope proteins, particles bearing no envelope and soluble monomeric gp120. Virology 366:245-262. 2007.
[PDF]
Iyer, S. P. N., Franti, M., Krauchuk, A. A., Fisch, D. N., Ouattara, A. A., Roux, K. H., Krawiec, L., Dey, A. K., Beddows, S., Maddon, P. J., Moore, J. P., and Olson, W. C. Purified, proteolytically mature HIV type 1 SOSIP gp140 envelope trimers. AIDS Res. Human Retroviruses 23:817-828. 2007.
[PDF]
Roux, K. H. and Taylor, K. A. AIDS virus envelope spike structure. Current Opinion in Structural Biology. 17: 244-252. 2007.
[PDF]
Zhu, P. Liu,J., Bess Jr., J., Chertova, E., Lifson, J. D., Grise, H., Ofek, G., Taylor, K. A., and Roux, K. H. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature, 441:847-852, 2006.
[PDF]
Moore,.P. L., Crooks, E. T. Porter, L, Zhu, P., Cayanan, C. S., Grise, H, Corcoran, P., Zwick, M. B., Franti, M., Morris, L., Roux, K. H.,. Burton, D. R., and Binley, J. M. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type-1. J. Virol. 80:2515-2528, 2006.
[PDF]
Pancera, M., Lebowitz, J., Schön, A., Zhu, P., Freire, E., Kwong, P. D., Roux, K. H., Sodroski, J. and Wyatt, R. Soluble mimetics of human immunodeficiency virus type 1 viral spikes produced by replacement of the native trimerization domain with a heterologous trimerization motif: characterization and ligand binding analysis. J. Virol., 79:9954-9969, 2005.
[PDF]
Roux, K. H., Zhu, P. Seavy, M., Katinger, H., Kunert, R., Seamon, V. Electron microscopic and immunochemical analysis of the broadly neutralizing HIV-1-specific, anti-carbohydrate antibody, 2G12. Mol. Immunol. 41:1001-1011. 2004.
[PDF]
Zhu, P., Chertova, E., Bess, J., Jr., Lifson, J. D., Arthur, L. O., Liu, J., Taylor, K. A., and Roux, K. H. Electron tomography analysis of envelope glycoprotein trimers on HIV and SIV virions. Proc. Natl. Acad. Sci. USA, 100:15812-15817. 2003.
[PDF]
Calarese, D. A., C. N. Scanlan, M. B. Zwick, S. Deechongkit, Y. Mimura, R. Kunert, P. Zhu, M. R. Wormald, R. I. Stanfield, K. H. Roux, J. W. Kelly, P. M. Rudd, R. A. Dwek, H. Katinger, D. R. Burton, and I. A. Wilson. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065-2071. 2003.
[PDF]
Immunoglobulin Structure and Immune Complex Publications
Lupinek, C., Roux, K. H., Laffer, S., Rauter, I., Reginald, K., Kneidinger, M., Blatt, K., Ball, Pree, I., Jahn-Schmid, B. Allam, J-P., Novak, N., T., Drescher, A., Kricek, F., Valent, P., Englund, H., and Valenta, R. Trimolecular complex formation of IgE, FceRI, and a recombinant nonanaphylactic single-chain antibody fragment with high affinity for IgE. J. Immunol. 182:4817-4829. 2009.
[PDF]
Herrin, B. H., Alder, M. N., Roux, K. H., Sina, C., Ehrhardt, G. R., Boydstron, J. A., Turnbough C. L. Jr., and Cooper, M. D. Structure and specificity of lamprey monoclonal antibodies. PNAS 105:2040-2045. 2008.
[PDF]
Roux, K. H., Zhu, P. Seavy, M., Katinger, H., Kunert, R., Seamon, V. Electron microscopic and immunochemical analysis of the broadly neutralizing HIV-1-specific, anti-carbohydrate antibody, 2G12. Mol. Immunol. 41:1001-1011, 2004.
[PDF]
Løset, G. Å., Roux, K. H., Zhu, P., Michaelsen, T. E., and Sandlie, I. Differential segmental flexibility and reach dictate the antigen binding mode of chimeric IgD and IgM: implications for the function of the B cell receptor. J. Immunol. 172:2925-2934, 2004.
[PDF]
Calarese, D. A., Scanlan, C. N., Zwick, M. B., Deechongkit, S., Mimura, Y., Kunert, R., Zhu, P., Wormald, M. R., Stanfield, R. L., Roux, K. H., Kelly, J. W., Rudd, P. M., Dwek, R. A., Katinger, H., Burton, D. R., and Wilson, I. A. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:20652071, 2003.
[PDF]
Johansson, A., Erlandsson, A., Eriksson, D., Ullen, A., Holm, P., Sundstrom, B. E., Roux, K. H., and Stigbrand, T. Idiotypic-anti-idiotypic complexes and their in vivo metabolism. Cancer 94:13061313, 2002.
[PDF]
Laffer, S., Hogbom, E., Roux, K. H., Sperr, W. R., Valent, P., Bankl, H. C., Vangelista, L., Kricek, F., Kraft, D., Gronlund, H., and Valenta, R. A Molecular model of type I allergy:identification and characterization of a nonanaphylactic anti-human IgE antibody fragment that blocks the IgE-Fc epsilon RI interaction and reacts with receptor-bound IgE. J. Allergy Clin. Immunol. 108:409416, 2001.
[PDF]
Roux, K. H., Greenberg, A. S., Green, L., Strelets, L., Avila, D., McKinney, E. C., and Flajnik. M. F. Structural analysis of the nurse shark (new) antigen receptor (NAR): molecular convergence of NAR and unusual mammalian immunoglobulins. Proc. Natl. Acad. Sci. USA 95:1180411809, 1998.
[PDF]
Roux, K. H., Strelets, L., Brekke, O. H., Sandlie, I., and Michaelsen, T. E. Comparisons of the ability of human IgG3 hinge mutants, IgM, IgE and IgA2 to form small immune complexes: a role for flexibility and geometry. J. Immunol. 161:40834090, 1998.
[PDF]
Roux, K. H. Negative stain immunoelectron microscopic analysis of small macromolecules of immunologic significance. Methods 10:247256, 1996.
[PDF]
