Research and Professional Interests:
Ants are among the most important organisms found in mid- to low-latitude terrestrial ecosystems, often playing keystone roles. Their success is due largely to their highly social way of life; they live in colonies ranging in size from a few dozen to several hundred million individuals. In many ways, ant colonies function like superorganisms—tasks and physiological roles (including reproduction) are divided among the queen and several types and ages of workers. My research recognizes the organism-like nature of ant colonies: each colony most commonly begins life as a single individual and goes through a process of growth, development, and death; specific colony functions are carried out by specialized classes of workers, larvae, or sexual individuals; competition takes place among colonies, just as it does among individuals of nonsocial animals; nourishment must be taken in by colonies, distributed, and allocated among colony members and colony functions; colonies must produce specialized sexual individuals (propagules) for reproducing themselves, and these must leave the colony, mate, and found new colonies.
Research Areas No Longer Active
Chemical defenses and natural history of tenebrionid beetles, including the evolution of defensive systems, and the evolution of life history characteristics.
Videos
https://www.youtube.com/watch?v=aYA4_UdYbLo
https://youtu.be/_-TbUleUYXE
Annotated research areas and publications
(1) General and Review: Advocacy for more systematic collection of social insect data and emphasis on natural history and life cycle studies.
Tschinkel, W.R. and E.O. Wilson (2014). Scientific Natural History: Telling the Epics of Nature. BioScience doi: 10.1093/biosci/biu033
Tschinkel, W.R. (2010) Back to basics: sociometry and sociogenesis of ant societies (Hymenoptera: Formicidae). Myrmecol. News 14:49-54, online
http://www.myrmecologicalnews.org/cms/images/pdf/volume14/mn14_49-54_non-printable.pdf
Tschinkel, W.R. 2006). The Fire Ants, Belknap /Harvard University Press, (730 pp.
Tschinkel, W.R. 1998) The reproductive biology of fire ant societies. Bioscience 48:593-605 (
Tschinkel, W. R. (1991). Insect sociometry: a field in search of data. Insectes Soc. 38: 77‑82
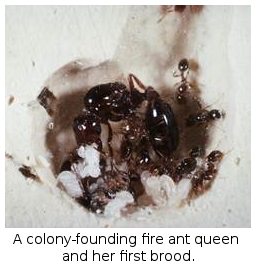 (2) Colony founding: Years of experimentation and survey, demonstrated unexpected complexity in the process of colony founding by newly-mated fire ant queens. Myriad interactions occur within founding colonies, including cannibalism of both queens and larvae. After the claustral period, brood raiding (brood stealing contests among newly-founded neighboring colonies) under natural conditions shapes the population dynamics of colony founding and early colony growth.
(2) Colony founding: Years of experimentation and survey, demonstrated unexpected complexity in the process of colony founding by newly-mated fire ant queens. Myriad interactions occur within founding colonies, including cannibalism of both queens and larvae. After the claustral period, brood raiding (brood stealing contests among newly-founded neighboring colonies) under natural conditions shapes the population dynamics of colony founding and early colony growth.
Tschinkel, W.R. (1998) An experimental study of pleometrotic colony-founding in the fire ant Solenopsis invicta: what is the basis for association? Behav. Ecol. Sociobiol. 43:247-257
Adams, E.S. and W.R. Tschinkel. (1995) Effects of foundress number on brood raids and queens survival in the fire ant Solenopsis invicta. Behav. Ecol. Sociobiol. 37:233-242
Tschinkel, W. R. (1993 )Resource allocation, brood production and cannibalism during colony founding in the fire ant, Solenopsis invicta. Behav. Ecol. Sociobiol. 33:209-223
Tschinkel, W. R. (1992). Brood raiding and the population dynamics of founding and incipient colonies of the fire ant, Solenopsis invicta. Ecol. Entomol. 17: 179‑188
Porter, S. D. and W. R. Tschinkel. (1988). Adaptive value of nanitic workers in newly founded red imported fire ant colonies (Hymenoptera: Formicidae). Ann. Entomol. Soc. Amer. 79: 723‑726
Tschinkel, W. R. and D. F. Howard.(1983) Pleometrotic colony foundation in the fire ant, Solenopsis invicta. Behav. Ecol. Sociobiol., 12: 103‑113.
 (3) A new mode of colony founding in ants. The majority of ants start new colonies by sending out newly mated queens, who start new colonies from reserves stored in their bodies, without the aid of workers. In addition to this well-known mode, fire ants also employ analternate mode of founding in which newly-mated queens seek out orphaned mature colonies. They enter these and exploit the worker force to help them start their own colony, making this a form of intraspecific social parasitism. Nothing like this has ever been describedbefore. It is likely that this phenomenon is widespread throughout the ants, but has heretofore simply never been detected.
(3) A new mode of colony founding in ants. The majority of ants start new colonies by sending out newly mated queens, who start new colonies from reserves stored in their bodies, without the aid of workers. In addition to this well-known mode, fire ants also employ analternate mode of founding in which newly-mated queens seek out orphaned mature colonies. They enter these and exploit the worker force to help them start their own colony, making this a form of intraspecific social parasitism. Nothing like this has ever been describedbefore. It is likely that this phenomenon is widespread throughout the ants, but has heretofore simply never been detected.
DeHeer, C.J. and W.R. Tschinkel. (1998) The success of alternative reproductive tactics in monogyne populations of the ant Solenopsis invicta: significance for transitions in social organization. Behav. Ecol. 9:130-135
Tschinkel, W.R. (1996) A newly-discovered mode of colony founding among fire ants. Ins. Soc. 43:267-276
McInnes, D.A. and W.R. Tschinkel. (1995)Queen dimorphism and reproductive strategies in the fire ant, Solenopsis geminata. Behav. Ecol. Sociobiol., 36:367-376
 (4) Social control of queen fertility. There is only one individual in a fire ant colony that lays eggs, and that is the queen. The rate at which she lays eggs must be coordinated with the size of her work force. Through experiments, we discovered and described themechanism that coordinates the queen’s egg-laying rate with the available brood-rearing labor, showing that the metamorphosing larvae are the key to this regulation.
(4) Social control of queen fertility. There is only one individual in a fire ant colony that lays eggs, and that is the queen. The rate at which she lays eggs must be coordinated with the size of her work force. Through experiments, we discovered and described themechanism that coordinates the queen’s egg-laying rate with the available brood-rearing labor, showing that the metamorphosing larvae are the key to this regulation.
Tschinkel, W.R. (1995) Stimulation of fire ant queen fecundity by a highly specific brood stage. Ann. Entomol. Soc. Amer. 88:876-882
Tschinkel, W. R. (1987).Social regulation of egg laying rate in queens of the fire ant, Solenopsis invicta. Physiol. Entomol. 13:237-250
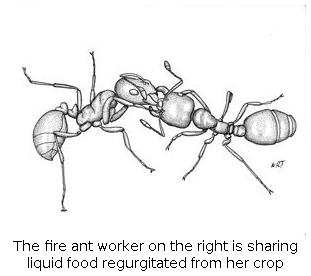 (5) Food traffic. Among ants, only the oldest workers leave the nest to forage. They bring liquid food back to the nest in their crops and share it with nestmates through a complex web of regurgitation. We illuminated the quantitative movement of food within the colony, showed that the global patterns result from local decisions based on local information, and discovered the “rules” by which food is shared with larvae and other workers. This is also the first detailed study of the involvement of larvae in the colony’s food web.
(5) Food traffic. Among ants, only the oldest workers leave the nest to forage. They bring liquid food back to the nest in their crops and share it with nestmates through a complex web of regurgitation. We illuminated the quantitative movement of food within the colony, showed that the global patterns result from local decisions based on local information, and discovered the “rules” by which food is shared with larvae and other workers. This is also the first detailed study of the involvement of larvae in the colony’s food web.
Cassill, D. L. and W. R. Tschinkel. (2000) Behavioral and developmental homeostasis in the fire ant, Solenopsis invicta. J. Insect Physiol. 46: 933-939
Cassill, D.L. and W.R. Tschinkel. (1999) Food flow and information flow in colonies of the fire ant, Solenopsis invicta. Book chapter in Information Processing in Social Insects, Detrain and Deneubourg, eds., Birkhauser Verlag, Basel. pp. 69-81
Cassill, D. L., and W. R. Tschinkel. (1995) Allocation of liquid food to larva via trophallaxis in the fire ant, Solenopsis invicta. Anim. Behav., 50:801-813
Cassill, D. and W.R. Tschinkel. (1996) A duration constant for worker-to-larva trophallaxis in fire ants. Ins. Soc. 43:149-166
Cassill, D.L. and W.R. Tschinkel. (1999). Task selection by workers of the fire ant, Solenopsis invicta. Behav. Ecol. Sociobiol. 45:301-310
Cassill, D.L. and W.R. Tschinkel. (1999) Regulation of diet in the fire ant, Solenopsis invicta. J. Insect Behav. 12:307-328
Cassill, D.L. and W.R. Tschinkel.(1999) Colony level effects on larval feeding in the fire ant, Solenopsis invicta. Insectes Sociaux. 46: 261-266.
Howard, D. F. and W. R. Tschinkel. (1981).Flow of food in colonies of the fire ant, Solenopsis invicta: A multi‑factorial approach. Physiol. Entomol., 6: 297‑306
Howard, D. F. and W. R. Tschinkel. (1981).Internal storage of liquid food in isolated workers of the fire ant, Solenopsis invicta. J. Insect Physiol., 27: 67‑74
Glunn, F. J., D. F. Howard and W. R. Tschinkel. (1981). Food preference in colonies of the fire ant, Solenopsis invicta. Insectes Sociaux, 28: 217‑222
Howard, D. F. and W. R. Tschinkel. (1980).The effect of colony size and starvation on food flow in the fire ant, Solenopsis invicta. Behav. Ecol. Sociobiol., 7: 293‑300
 (6) Evolution of colony life histories: The methods of sociometry/ sociogenesis, provide a simple procedure for the simultaneous determination of suites of colony characteristics and how they develop during colony growth. This is the social insect counterpart of embryonic development, and has received little attention. Sociometric/sociogenic studies on fire ants and harvester ants produced the first quantitative, full life-history descriptions of an ant colony, from birth to death.
(6) Evolution of colony life histories: The methods of sociometry/ sociogenesis, provide a simple procedure for the simultaneous determination of suites of colony characteristics and how they develop during colony growth. This is the social insect counterpart of embryonic development, and has received little attention. Sociometric/sociogenic studies on fire ants and harvester ants produced the first quantitative, full life-history descriptions of an ant colony, from birth to death.
C.R. Smith and W.R. Tschinkel, (2006). The sociometry and sociogenesis of reproduction in the Florida harvester ant (Pogonomyrmex badius) J. Insect Sci. 6:32 http://www.insectscience.org/6.32/
Tschinkel, W.R. (1999).Sociometry and sociogenesis of colony-level attributes of the Florida harvester ant (Hymenoptera: Formicidae). Ann. Entom. Soc. Amer. 92:80-89
Tschinkel, W.R. (1998). Sociometry and sociogenesis of colonies of the harvester ant, Pogonomyrmex badius:: worker characteristics in relation to colony size and season. Ins. Soc. 45:385-410
Tschinkel, W.R. (1998). Sociometry and sociogenesis of colonies of the harvester ant, Pogonomyrmex badius, in relation to colony size and season: distribution of workers and seeds in the nest. Insectes Sociaux. 45:385-410
Tschinkel, W. R. (1993).Sociometry and sociogenesis in colonies of the fire ant, Solenopsis invicta during one annual cycle.. Ecol. Monogr63:425-457
Tschinkel, W. R. (1991). Insect sociometry: a field in search of data. Insectes Soc. 38: 77‑82
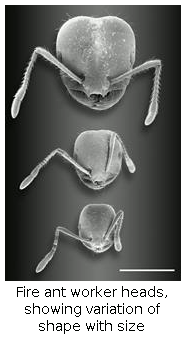 (7) Worker polymorphism. Workers in fire ant colonies vary about 15-fold in weight from the smallest to the largest. This so-called polymorphism develops gradually as colonies grow. Detailed descriptions of polymorphism of colonies of a full range of sizes reveals how polymorphism arises during colony development. Polymorphism has been hypothesized to make colonies more efficient and therefore to increase their fitness. We tested this in experiments that used brood rearing efficiency as a surrogate of fitness.
(7) Worker polymorphism. Workers in fire ant colonies vary about 15-fold in weight from the smallest to the largest. This so-called polymorphism develops gradually as colonies grow. Detailed descriptions of polymorphism of colonies of a full range of sizes reveals how polymorphism arises during colony development. Polymorphism has been hypothesized to make colonies more efficient and therefore to increase their fitness. We tested this in experiments that used brood rearing efficiency as a surrogate of fitness.
Tschinkel, W.R. (2013) The morphometry of Solenopsis fire ants. PlosOne 8(11): e79559. doi:10.1371/journal.pone.0079559.
Araujo, M. and W. R. Tschinkel (2010). Worker allometry in relation to colony size and social form in the fire ant, Solenopsis invicta, J. Insect Sci. 10:94 http://jinsectscience.oxfordjournals.org/content/10/1/94
Tschinkel W.R., A. Mikheyev and S. Storz. (2003) The allometry of worker polymorphism in the fire ant, Solenopsis invicta. J. Insect Sci. 3:2
Tschinkel, W. R. (1988) Colony growth and the ontogeny of worker polymorphism in the fire ant, Solenopsis invicta Buren. Behav. Ecol. Sociobiol. 22: 103‑115.
Porter, S. D. and W. R. Tschinkel. (1985).Fire ant polymorphism: the ergonomics of brood production. Behav. Ecol. Sociobiol. 16: 323‑336.
Porter, S. D. and W. R. Tschinkel. (1985). Fire ant polymorphism: factors affecting worker size. Ann. Entomol. Soc. Amer. 78: 381‑386.
Wood, L. A. and W. R. Tschinkel. (1981) Quantification and modification of worker size variation in the fire ant, Solenopsis invicta. Insectes Sociaux, 28: 117‑128
(8) Population biology of fire ant colonies. Ant populations consist of populations of colonies, as well as individual ants. There is thus an additional level of population processes in social insects, and these have rarely been studied. A five-year study of the population biology of fire ant colonies included the appearance, growth and decline of colonies, competitive territorial interactions, migration, the use of space and the relationship of territory size, colony size and neighborhoods.

A fire ant territory: boundaries shown by tape, colony by blue flag.
Adams, E.S. and W.R. Tschinkel. (2001). Mechanisms of population regulation in the fire ant Solenopsis invicta: an experimental study, J. Anim. Ecol. 70:355-369
Adams, E. S., and W. R. Tschinkel. (1995). Density‑dependent competition in fire ants: effects on colony survivorship and size variability. J. Anim. Ecol. 64:315-324
Adams, E.S. and W.R. Tschinkel. (1995). Spatial dynamics of colony interactions in young populations of the fire ant, Solenopsis invicta. Oecologia, 102:156-163
Tschinkel, W. R., E. S. Adams, and T. Macon. (1995). Territory area and colony size in the fire ant, Solenopsis invicta, J. Anim. Ecol., 64:473-480
 (9) Fire ant, native ant interactions. The fire ant is an exotic invasive ant from Brazil that is of great political and economic importance. Ecological work on this species has clearly demonstrated that ecological disturbance favors the establishment and existence of this “weedy” species, making humans the fire ant’s best friend. Moreover, the long-held belief that fire ants suppress native ant populations was shown to be false.
(9) Fire ant, native ant interactions. The fire ant is an exotic invasive ant from Brazil that is of great political and economic importance. Ecological work on this species has clearly demonstrated that ecological disturbance favors the establishment and existence of this “weedy” species, making humans the fire ant’s best friend. Moreover, the long-held belief that fire ants suppress native ant populations was shown to be false.
King, J.R. and W. R. Tschinkel (2013) Experimental evidence that human impacts drive fire ant invasions and ecological change: Response. J. Anim. Ecol., in press.
Tschinkel, W.R. and King, J.R. (2013) The role of habitat in the persistence of fire ant (Solenopsis invicta) populations. PlosOne 8(10): e78580. doi:10.1371/journal.pone.0078580.
King, J.R. and W. R. Tschinkel (2013) Experimental evidence for weak effects of fire ants in a naturally invaded pine-savanna ecosystem in north Florida. Ecol. Entomol. 38: 68–75
King, J.R., W. R. Tschinkel and K.G. Ross (2009) A case study of human exacerbation of the invasive species problem: transport and establishment of polygyne fire ants in Tallahassee, Florida, USA. Biol. Invasions 11:373-377.
King, J.R. and W. R. Tschinkel (2008). Experimental evidence that human impacts drive fire ant invasions and ecological change. PNAS 105:20339-20343.
Tschinkel, W.R. and J.R. King (2007). Targeted removal of ant colonies in ecological experiments, using hot water. Journal of Insect Science, online athttp://jinsectscience.oxfordjournals.org/content/7/1/41
Tschinkel, W.R. and J.R. King, (2007). Targeted removal of ant colonies in ecological experiments, using hot water. Journal of Insect Science, online athttp://jinsectscience.oxfordjournals.org/content/7/1/41
King, J.R. and W.R. Tschinkel, (2006). Experimental evidence that the introduced fire ant, Solenopsis invicta, does not competitively suppress co-occurring ants in a disturbed habitat, J. Animal Ecol. 75: 1370–1378
Lubertazzi, D. and W.R. Tschinkel. (2003).Ant community change across a ground vegetation gradient in north Florida's longleaf pine flatwoods. J. Insect Science 3:21
Tschinkel, W.R. (1998) The reproductive biology of fire ant societies. Bioscience 48:593-605
Tschinkel, W. R. (1993).The fire ant: still unvanquished. Proc. Indiana Acad. Sci., in Biological Pollution: The Control and Impact of Invasive Exotic Species, ed. B.N. McKnight, Indiana Acad. Sci., Indianapolis.
Tschinkel, W. R. (1988).Distribution of the fire ants Solenopsis invicta and S. geminata in north Florida in relation to habitat and disturbance. Ann. Entomol. Soc. Amer. 81: 76‑81.
Tschinkel, W. R. (1986) The ecological nature of the fire ant, some aspects of colony function and some unanswered questions, in Fire Ants and Leaf‑cutter Ants: Biology and Management, C. S. Lofgren and R. K. Vander Meer, eds. Westview Press, 1986.
 (10) Other fire ant biology. In spite of the importance of fire ants, much of their biology is unknown or poorly understood. A number of our papers illuminate important aspects of the biology of this ant, including its capacity to regulate larval development by choosing developmental temperatures, the longevity of its queens and colonies, its sanitation behavior, its brood-care signals, sperm storage, food storage, organization of foraging and the effect of temperature on foraging.
(10) Other fire ant biology. In spite of the importance of fire ants, much of their biology is unknown or poorly understood. A number of our papers illuminate important aspects of the biology of this ant, including its capacity to regulate larval development by choosing developmental temperatures, the longevity of its queens and colonies, its sanitation behavior, its brood-care signals, sperm storage, food storage, organization of foraging and the effect of temperature on foraging.
Tschinkel, W.R. (2010) The organization of foraging in the fire ant, Solenopsis invicta. J. Insect Sci. 10:26 online at http://jinsectscience.oxfordjournals.org/content/11/1/26
Gayahan, G.G, and W.R. Tschinkel (2008) Fire ants (Solenopsis invicta) dry and store insect pieces for later use, J. Insect Sci. online athttp://jinsectscience.oxfordjournals.org/content/8/1/39
Penick, C.A. and W. R. Tschinkel (2008) Thermoregulatory brood transport in the fire ant, Solenopsis invicta, Insectes Sociaux 55:175-172.
Tschinkel, W.R. and J.R. King (2007). Targeted removal of ant colonies in ecological experiments, using hot water. Journal of Insect Science, online athttp://jinsectscience.oxfordjournals.org/content/7/1/41
Haight, K.L. and W. R. Tschinkel. (2003) Patterns of venom synthesis and use in the fire ant, Solenopsis invicta. Toxicon 42:673-683
McInnes, D.A. and W.R. Tschinkel. (1995). Queen dimorphism and reproductive strategies in the fire ant, Solenopsis geminata. Behav. Ecol. Sociobiol., 36:367-376
Porter, S. D., and W. R. Tschinkel. (1993).Fire ant thermal preferences: behavioral control of growth and metabolism. Behav. Ecol. Sociobiol. 32:321-329.
Tschinkel, W. R. (1993). The fire ant: still unvanquished. Proc. Indiana Acad. Sci., in Biological Pollution: The Control and Impact of Invasive Exotic Species, ed. B.N. McKnight, Indiana Acad. Sci., Indianapolis
Tschinkel, W. R., and S. D. Porter. (1988). The efficiency of sperm use in queens of the fire ant, Solenopsis invicta Buren. Ann. Entomol. Soc. Amer. 81: 777‑781.
Porter, S. D., and W. R. Tschinkel. (1987).Foraging in Solenopsis invicta (Hymenoptera: Formicidae): effects of weather and season. Envir. Entomol. 16: 802‑808.
Tschinkel, W. R. (1987). Fire ant queen longevity and age: estimation by sperm depletion. Ann. Entomol. Soc. Amer. 80: 263‑266.
Tschinkel, W. R. and D. R. Howard. (1980) .A simple, non‑toxic home remedy against fire ants. J. Ga. Entomol. Soc. 15:102‑105
Howard, D. R. and W. R. Tschinkel. (1976).Aspects of necrophoric behavior in the red imported fire ant, Solenopsis invicta. Behaviour 56: 157‑180.
Walsh, J. P. and W. R. Tschinkel. (1974). Brood recognition by contact pheromone in the imported fire ant, Solenopsis invicta. Animal Behaviour 22: 695‑704.
 (11) Architecture of subterranean ant nests. We have pioneered the study of nest architecture of ground-nesting ants through the creation of nest casts using orthodontal plaster or liquid metal. This work is still in progress, but has received national and international attention. The initial goal of this research is to provide an inventory of the structures ants create, as well as an understanding of how colonies are organized to produce these structures.
(11) Architecture of subterranean ant nests. We have pioneered the study of nest architecture of ground-nesting ants through the creation of nest casts using orthodontal plaster or liquid metal. This work is still in progress, but has received national and international attention. The initial goal of this research is to provide an inventory of the structures ants create, as well as an understanding of how colonies are organized to produce these structures.
Tschinkel W. R., Rink W. J., Kwapich C. L. (2015) Subterranean transport of sand and seeds by caching in the harvester ant, Pogonomyrmex badius, PlosOne (in press, July 2015).
Tschinkel, W.R. (2015) The architecture of subterranean ant nests: beauty and mystery underfoot. Journal of Bioeconomics. In press.
Tschinkel WR (2015) Biomantling and bioturbation by colonies of the Florida harvester ant, Pogonomyrmex badius. PLoS One 10(3): e0120407. doi:10.1371/journal.pone.0120407
Tschinkel WR (2014) Nest relocation and excavation in the Florida harvester ant, Pogonomyrmex badius. PLoS One 9(11): e112981. doi:10.1371/journal.
Tschinkel, W.R. (2013). Florida harvester ant nest architecture, nest relocation and soil carbon dioxide gradients. Online at PlosOne 8(3): e59911. doi:10.1371/journal.pone.0059911. or http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0059911
Tschinkel, W.R. (2013) A method for using ice to construct subterranean ant nests (Hymenoptera: Formicidae) and other soil cavities. Myrmecol. News 18: 99-102.
Rink W. J, Dunbar, J.S., Tschinkel W.R., Kwapich C., Repp A., Stanton W. and Thulman, D.K. (2013) Subterranean transport and deposition of quartz by ants in sandy sites relevant to age overestimation in optical luminescence dating. J. Archeol. Sci. 40: 2217–2226
Tschinkel, W.R. (2011) The nest architecture of three species of Aphaenogaster in north Florida. J. Insect Sci. online: http://jinsectscience.oxfordjournals.org/content/11/1/105
Tschinkel, W. R. (2010) Methods for casting subterranean ant nests. J. Insect Sci. 10:88 online at: http://jinsectscience.oxfordjournals.org/content/10/1/88
Cerquera, L.M. and W.R. Tschinkel (2009). The nest architecture of Odontomachus brunneus. J. Insect Sci. 10:64. online at http://jinsectscience.oxfordjournals.org/content/10/1/64
Tschinkel, W.R. (2005) The Nest Architecture of the Ant, Camponotus socius. J. Insect Science 5:9 (available online at http://insectscience.org/5.9/).
Tschinkel, W.R. (2004). The Nest Architecture of the Florida Harvester Ant, Pogonomyrmex badius. J. Insect Science 4:21 (online at http://www.insectscience.org/4.21/
Mikheyev, A. and W. R. Tschinkel. (2004). Nest architecture of the ant Formica pallidefulva: structure, costs and rules of excavation. Insectes Sociaux 51:30-36
Tschinkel, W.R. (2003) Subterranean Ant Nest Architecture: Trace Fossils of the Future? Paleogeogr. Paleoclim. Paleoecol. 192:321-333
Cassill, D.L., W.R. Tschinkel and S.B. Vinson. (2002)Nest complexity, group size and brood rearing in the fire ant, Solenopsis invicta. Insectes Soc. 49(2):158-163
Tschinkel, W.R. (2001) Colonies in space. Nat. Hist.
Tschinkel, W.R. (1998).Sociometry and sociogenesis of colonies of the harvester ant, Pogonomyrmex badius, in relation to colony size and season: distribution of workers and seeds in the nest. Insectes Sociaux. 45:385-410
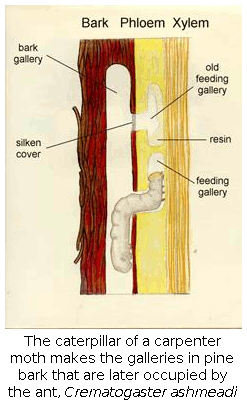 (12) Natural history of arboreal ants. With the discovery by Dr. Frances James and Charles Hess that the endangered red-cockaded woodpecker ate mostly a single species of tree-dwelling ant, Crematogaster ashmeadi, it became important to learn as much as possible about the ecology and natural history of this critical ant. Subsequently, we have worked out most of the basic outlines of the colony cycle, natural history, distribution and abundance of this ant. In the process, we discovered that this previously unknown ant is the most abundant and dominant arboreal ant in the coastal plain pine forests of the southern states.
(12) Natural history of arboreal ants. With the discovery by Dr. Frances James and Charles Hess that the endangered red-cockaded woodpecker ate mostly a single species of tree-dwelling ant, Crematogaster ashmeadi, it became important to learn as much as possible about the ecology and natural history of this critical ant. Subsequently, we have worked out most of the basic outlines of the colony cycle, natural history, distribution and abundance of this ant. In the process, we discovered that this previously unknown ant is the most abundant and dominant arboreal ant in the coastal plain pine forests of the southern states.
Tschinkel, W.R. (2002). The Natural History of the Arboreal Ant, Crematogaster ashmeadi (Hymenoptera: Formicidae). J. Insect Sci. 2:12
Baldacci, J. and W.R. Tschinkel. (1999).An experimental study of colony founding in pine saplings by queens of the arboreal ant, Crematogaster ashmeadi. Ins. Soc. 46:41-44
Tschinkel, W.R. and C. Hess. (1999)The arboreal ant community of a north Florida pine forest, with emphasis on Crematogaster ashmeadi. Ann. Entomol. Soc. Amer. 92:63-70
Hahn, D.A. and W.R. Tschinkel. (1997) Settlement and distribution of newly-mated queens of the arboreal ant, Crematogaster ashmeadi, in a longleaf pine forest. Ins. Soc. 44:323-336
 (13) Natural history of other ants. The natural history of most ant species is, at best, poorly known. Adding to the store of natural history knowledge allows inferences about the evolution of ant sociality, ecology and behavior to be drawn. A number of “background” projects on the natural history of local species of ants have revealed novel and fascinating things about the lives of Florida’s ants.
(13) Natural history of other ants. The natural history of most ant species is, at best, poorly known. Adding to the store of natural history knowledge allows inferences about the evolution of ant sociality, ecology and behavior to be drawn. A number of “background” projects on the natural history of local species of ants have revealed novel and fascinating things about the lives of Florida’s ants.
Mason, K. S., Kwapich, C. L., Tschinkel, W.R. (2015) Respiration, worker body size, tempo and activity in whole colonies of ants. Physiol. Entomol. (in press). DOI: 10.1111/phen.12099
Murdock, T. and W.R. Tschinkel (2015). Seasonal life history and nest architecture of the ant, Pheidole morrisi. Insectes Sociaux, DOI 10.1007/s00040-015-0403-9.
Tschinkel, W.R., T. Murdock, J.R. King and C. Kwapich (2012). Ants and groundwater in the north Florida flatwoods. Journal of Insect Science12:114. online: http://jinsectscience.oxfordjournals.org/content/12/1/114
Hart,L. M. and W. R. Tschinkel (2012) A seasonal natural history of the ant, Odontomachus brunneus. Insectes Sociaux. 59:45–54
Laskis, K.O. and W. R. Tschinkel (2009). The Seasonal Natural History of the Ant, Dolichoderus mariae (Hymenoptera: Formicidae) In Northern Florida, J. Insect Sci. Online http://jinsectscience.oxfordjournals.org/content/9/1/2
King, J.R. and W.R. Tschinkel (2007). Range expansion and local population increase of the exotic ant, Pheidole obscurithorax, in the southeastern United States. Florida Entomologist 90: 435-439.
Storz, S. and W.R. Tschinkel. (2003) Distribution, spread, and ecological associations of the introduced ant Pheidole obscurithorax in the southeastern United States. J. Insect Sci. 4:12 Available online: www.insectscience.org/
Tschinkel, W.R. (2002).The Natural History of the Arboreal Ant, Crematogaster ashmeadi (Hymenoptera: Formicidae). J. Insect Sci. 2:12
Powell, S. and W. R. Tschinkel. (1999) The dominance interactions of the ant, Odontomachus brunneus and the generation of polyethism. J. Insect Behav. 12:436-440
McInnes, D.A. and W.R. Tschinkel. (1996) Mermithid nematode parasitism of Solenopsis ants (Hymenoptera: Formicidae) of northern Florida. Ann. Entomol. Soc. Amer. 89:231-237
McInnes, D.A. and W.R. Tschinkel. (1995)Queen dimorphism and reproductive strategies in the fire ant, Solenopsis geminata. Behav. Ecol. Sociobiol., 36:367-376
Hood, W. G., and W. R. Tschinkel. (1990). Desiccation resistance in arboreal and terrestral ants. Physiol. Entomol. 15: 23‑35
Tschinkel, W. R. (1987). Seasonal life history and nest architecture of a cold‑loving ant, Prenolepis imparis. Insectes Soc. 34: 143‑164
Tschinkel, W. R. (1987). The relationship between ovariole number and spermathecal sperm count in ant queens: a new allometry. Ann. Entomol. Soc. Amer. 80: 208‑211
Tschinkel, W. R. and A. Bhatkar. (1974). Oriented mound‑building in the ant, Trachymyrmex septentrionalis. Environ. Entomol. 3: 667‑673
 (14) Harvester ants and fungus gardening ants. We have studied the natural history of some local ants more intensively than others. Two in particular stand out--- the Florida harvester ant, and the fungus gardening ant, Trachymyrmex septentrionalis. Each species does interesting things that invite explanation. The harvester ant collects seeds for food, but also collects charcoal pieces to decorate its nest (the detailed sociometry of this species was noted in item 5, above). The fungus-gardening ant collects plant bits and caterpillar droppings for its fungus gardens, raising the question, “does it know what is good for its fungus?”
(14) Harvester ants and fungus gardening ants. We have studied the natural history of some local ants more intensively than others. Two in particular stand out--- the Florida harvester ant, and the fungus gardening ant, Trachymyrmex septentrionalis. Each species does interesting things that invite explanation. The harvester ant collects seeds for food, but also collects charcoal pieces to decorate its nest (the detailed sociometry of this species was noted in item 5, above). The fungus-gardening ant collects plant bits and caterpillar droppings for its fungus gardens, raising the question, “does it know what is good for its fungus?”
Kwapich, C.M. and W. R. Tschinkel (2013) Demography, demand, death and the seasonal allocation of labor in the Florida harvester ant (Pogonomyrmex badius). Behav. Ecol. Sociobiol. 67:2011-2027. DOI: 10.1007/s00265-013-1611-9.
Seal, J.N. and W.R. Tschinkel (2010) Distribution of the fungus-gardening ant (Trachymyrmex septentrionalis) during and after a record drought. Insect Conservation and Diversity (2010) 3:134-142.
Seal, J.N. and W. R. Tschinkel (2008). Food limitation in the fungus gardening ant, Trachymyrmex septentrionalis. Ecol. Entomol. 33:597-607.
Seal, J.N. and W.R. Tschinkel, (2007) Energetics of newly mated queens and colony founding in the fungus-gardening ants Cyphomyrmex rimosus and Trachymyrmex septentrionalis (Hymenoptera: Formicidae). Physiological Entomology, 32: 8-15.
Seal, J.N. and W.R. Tschinkel (2007) Complexity in an obligate mutualism: Do fungus-gardening ants know what makes their garden grow? Behav. Ecol. Sociobiol. 61:1151–1160.
Seal, J.N. & Tschinkel, W.R. (2007) Co-evolution and the superorganism: switching cultivars does not alter the performance of fungus-gardening ant colonies. Functional Ecology, 21, 988-997.
Smith C. R. and W. R. Tschinkel, (2007). The adaptive nature of non-food collection for the Florida harvester ant, Pogonomyrmex badius. Ecological Entomology 32:105-112
Smith C.R. and W.R. Tschinkel, (2006) The sociometry and sociogenesis of reproduction in the Florida harvester ant (Pogonomyrmex badius) J. Insect Sci. 6:32. http://www.insectscience.org/6.32/
Seal J.N. and Tschinkel W.R. (2006) Energetics of newly mated queens and colony founding in the fungus-gardening ants Cyphomyrmex rimosus and Trachymyrmex septentrionalis (Hymenoptera: Formicidae). Physiol Entomol. 32: 8-15.
Seal, J.N. and W.R. Tschinkel, (2006). Complexity in an obligate mutualism: Do fungus-gardening ants know what makes their garden grow? Behav. Ecol. Sociobiol. 61:1151–1160.
Seal J.N. and Tschinkel W.R. (2006) Colony productivity of the fungus-gardening ant, Trachymyrmex septentrionalis McCook, in a Florida pine forest (Hymenoptera: Formicidae). Ann Entomol Soc Am: 99: 673-682
Smith C. R. and W. R. Tschinkel, (2005) Object depots in the genus Pogonomyrmex: Exploring the “who”, what, when, and where. J. Insect Behav. 18:859-879
 (15) Chemical defense, pheromones and systematics of tenebrionid and other beetles. Tenebrionid beetles are a cosmopolitan family of about 20,000 species, most of whom possess a chemical defensive system. We carried out the largest and most complete comparative study of this chemical defense system, including analysis of the chemical composition of the secretion, anatomy of the glands, and defensive behavior. This remains a standard-setting study that has rarely been equaled or surpassed. These comparative studies culminated in the revision of the higher systematics of family Tenebrionidae.
(15) Chemical defense, pheromones and systematics of tenebrionid and other beetles. Tenebrionid beetles are a cosmopolitan family of about 20,000 species, most of whom possess a chemical defensive system. We carried out the largest and most complete comparative study of this chemical defense system, including analysis of the chemical composition of the secretion, anatomy of the glands, and defensive behavior. This remains a standard-setting study that has rarely been equaled or surpassed. These comparative studies culminated in the revision of the higher systematics of family Tenebrionidae.
Hill, C. S. and W. R. Tschinkel. (1985). Defensive secretion production in the tenebrionid beetle, Zophobas atratus: effects of age, sex and milking frequency. J. Chem. Ecol. 11: 1083‑1092
Doyen, J. T. and W. R. Tschinkel. (1982). Phenetic and cladistic relationships among tenebrionid beetles (Coleoptera). System. Entomol. 7: 127‑183
Blum, M. S., T. H. Jones, G. J. House and W. R. Tschinkel. (1981) Defensive secretions of tiger beetles: cyanogenetic basis. Comp. Biochem. Physiol., 69B: 903‑904
Tschinkel, W. R. and J. T. Doyen. (1980).Comparative anatomy of the defensive gland, ovipositor and female genital tube of the tenebrionid beetles. Intern. J. Insect Morphol. Embryol. 9: 321‑369
Lloyd, H S., S. L. Evans, A. H. Khan, W. R. Tschinkel and M. S. Blum. (1978). 8‑Hydroxyisocoumarin and 3,4‑dihydro‑8‑hydroxyisocoumarin in the defensive secretion of the tenebrionid beetle, Apsena pubescens. Insect Biochem. 8: 333‑336
Eisner, T., T. H. Jones, D. J. Aneshansley, W. R. Tschinkel, R. E. Silberglied and J. Meinwald. (1977). Chemistry of defensive secretions of bombardier beetles (Brachinini, Metriini, Ozaenini, Paussini). J. Insect Physiol. 23: 1383‑1386
Tschinkel, W. R. (1975).A comparative study of the chemical defensive system of tenebrionid beetles. I. Chemistry of the secretion. J. Insect Physiol. 21: 753‑783
Tschinkel, W. R. (1975).A comparative study of the chemical defensive system of tenebrionid beetles. III. Morphology of the glands. J. Morphol. 145: 355‑370
Tschinkel, W. R. (1975).A comparative study of the chemical defensive system of tenebironid beetles. II. Defensive behavior and ancillary features. Ann. Entomol. Soc. Amer. 68: 439‑453
Tschinkel, W. R. (1975) Unusual occurrence of aldehydes and ketones in the defensive secretion of the tenebrionid beetle, Eleodes beameri. J. Insect Physiol. 21: 659‑671
Tschinkel, W. R. (1972) .6‑Alkylated‑naphthoquinones from defensive secretion of the tenebrionid beetle, Argoporis alutacea. J. Insect Physiol. 18: 711‑722
Tschinkel, W. R. (1970) Chemical studies on the sex pheromone of Tenebrio molitor (Coleoptera: Tenebrionidae). Ann. Ent. Soc. Amer. 63: 626‑627
Tschinkel, W. R. (1969) Phenols and quinones from the defensive secretion of the tenebrionid beetle, Zophobas rugipes. J. Insect Physiol. 15: 191‑200
Tschinkel, W. R., C. D. Willson and H. A. Bern. (1967) Sex pheromone of the mealworm beetle (Tenebrio molitor). J. Exp. Zool. 164: 81‑86
 (16) Inhibition of metamorphosis by crowding. Crowding in tenebrionid beetle larvae has a novel effect in that it directly inhibits metamorphosis through the effects of larval-larval contacts. We worked out its natural history, and through experimentation, described the mechanism of the inhibition and its life-history consequences in Zophobas atratus. WE also demonstrated the importance of this effect in natural populations.
(16) Inhibition of metamorphosis by crowding. Crowding in tenebrionid beetle larvae has a novel effect in that it directly inhibits metamorphosis through the effects of larval-larval contacts. We worked out its natural history, and through experimentation, described the mechanism of the inhibition and its life-history consequences in Zophobas atratus. WE also demonstrated the importance of this effect in natural populations.
Tschinkel, W. R. (1993) Crowding, maternal age and age at pupation in the life history of Zophobas rugipes (Coleoptera: Tenebironidae). Ann. Entomol. Soc. Amer. 86:278-297
Tschinkel, W. R. (1981).Larval dispersal and cannibalism in natural population of Zophobas atratus (Coleoptera: Tenebrionidae). Anim. Behavior 29:990‑996
Tschinkel, W. R. (1978). Dispersal behavior of the larval tenebrionid beetle, Zophobas rugipes. Physiol. Zool. 51: 300‑313
Tschinkel, W. R. and G. van Belle. (1976).Dispersal of larvae of the tenebrionid beetle, Zophobas rugipes, in relation to weight and crowding. Ecology 57: 161‑168
Tschinkel, W. R. and C. D. Willson. (1971). Inhibition of pupation due to crowding in some tenebrionid beetles. J. Exp. Zool. 176: 137‑146
(17) Causes and characteristics of Namibian fairy circles. The Namib Desert of The eastern margin of the Namib Desert of Namibia, Africa is home to a mysterious phenomenon in which the grassy vegetation is punctuated by tens of thousands of evenly spaced barren spots surrounded by taller grass. The origin of these circles is unknown, although many hypotheses have been offered. Using satellite images and visits on the ground, I am describing the range of variation of fairy circles and estimating their life span.

Fairy circles in the Namib Desert of Namibia.
Tschinkel W.R. (2015) Experiments testing the causes of Namibian fairy circles. PloS One (submitted April 2015).
Tschinkel, W.R. (2012) The life cycle and life span of Namibian fairy circles. PLoS ONE 7(6): e38056. doi:10.1371/journal.pone.0038056 . Online at: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0038056
Tschinkel, W. R. (2010) The foraging tunnel system of the Namibian termite, Baucaliotermes hainesi. J. Insect Sci. 10:65. online at: http://jinsectscience.oxfordjournals.org/content/10/1/65
Odd and Ends
Occasional opportunistic research into unusual areas that are not the normal focus of our research.
Nierenberg, N, W. R. Tschinkel and V. J. Tschinkel (2010). Early Climate Change Consensus at the National Academy: The Origins and Making of Changing Climate, Historical Studies in the Natural Sciences, 40 (3):318–349
Tschinkel, W. R. (1984) .Zophobas rugipes and Z. atratus are the same species. Coleop. Bull. 38: 325‑333
Tschinkel, W. R. (1978). Ovoviviparity in some tenebrionid beetles. Coleopt. Bull. 32: 315‑318
Tschinkel, W. R. and J. T. Doyen. (1976). Sound production by substratal tapping in the tentyriid beetles of the genus Eusattus. Coleopt. Bull. 30: 331‑335
Doyen, J. T. and W. R. Tschinkel. (1974). Population size, microgeographic distribution and habitat separation in some tenebrionid beetles (Coleoptera). Ann. Entomol. Soc. Amer. 67: 617‑626
Tschinkel, W. R. and P. G. Close. (1973). The trail pheromone of the termite, Trinervitermes trinervoides. J. Insect Physiol. 19: 707‑721
Tschinkel, W. R. (1973). The sorption of water vapor by windborne plant debris in the Namib Desert. Madoqua II 2: 21‑24
The Fire Ants (Harvard/Belknap Press, 2006). This large volume brings together and interprets all current knowledge of fire ant biology for both the professional biologist and the educated lay reader. It was nominated for a Pulitzer Prize in September 2006.

Dust jacket of The Fire Ants.
From Reviews of The Fire Ants
- In his foreword to the book, E.O. Wilson wrote "Tschinkel has delivered a masterpiece" and went on to say that "this is how future biology will be written: a holistic account of a species or group of species across all levels of biological organization…”
- James Gorman, NY Times science writer, in an extremely positive review, said, "This is what the public needs to know about science, not just the results presented in the driest form possible."
- The Times Literary Supplement (London) wrote: "This book is a masterly and detailed account of some of nature's greatest opportunists, the fire ants." Referring to the anecdotal and less-technical "interludes" sprinkled throughout the book, the TLS called them "highly readable primers… that give the flavour of the man beautifully", and then went on to write, "No doubt, the stuffed shirt faction of academic entomologists will harrumph and snort at the sometimes folksy style, but that is their problem.”
- “This is a wonderful book, comprehensive in its coverage of fire ant social biology, extraordinarily lucid in its description of complex topics, and beautifully synthetic in tying together the disparate threads of evidence relevant to the discussion of each topic.”
- “This book is without parallel as a thorough description of the biology of an important social insect.”
- “Not only is the author, Walter Tschinkel, an expert on Fire Ants, he is a skillful writer. His ability to sprinkle humor in every chapter makes the book enjoyable even to the non-scientific reader. He will expel many false claims of the Fire Ant menace and enlighten the reader with facts gathered from over 30 years of observations and experiments.”
- “There are lovely essays on the behavior of ant researchers interspersed among the more numerous and scientifically dense chapters. It is really rather astonishing all that Tschinkel and his fellows have been able to ask the ants experimentally and get them to reply… Tschinkel's volume is a beautiful monument to fire ants and to science.
 (3) A new mode of colony founding in ants. The majority of ants start new colonies by sending out newly mated queens, who start new colonies from reserves stored in their bodies, without the aid of workers. In addition to this well-known mode, fire ants also employ analternate mode of founding in which newly-mated queens seek out orphaned mature colonies. They enter these and exploit the worker force to help them start their own colony, making this a form of intraspecific social parasitism. Nothing like this has ever been describedbefore. It is likely that this phenomenon is widespread throughout the ants, but has heretofore simply never been detected.
(3) A new mode of colony founding in ants. The majority of ants start new colonies by sending out newly mated queens, who start new colonies from reserves stored in their bodies, without the aid of workers. In addition to this well-known mode, fire ants also employ analternate mode of founding in which newly-mated queens seek out orphaned mature colonies. They enter these and exploit the worker force to help them start their own colony, making this a form of intraspecific social parasitism. Nothing like this has ever been describedbefore. It is likely that this phenomenon is widespread throughout the ants, but has heretofore simply never been detected.  (2) Colony founding: Years of experimentation and survey, demonstrated unexpected complexity in the process of colony founding by newly-mated fire ant queens. Myriad interactions occur within founding colonies, including cannibalism of both queens and larvae. After the claustral period, brood raiding (brood stealing contests among newly-founded neighboring colonies) under natural conditions shapes the population dynamics of colony founding and early colony growth.
(2) Colony founding: Years of experimentation and survey, demonstrated unexpected complexity in the process of colony founding by newly-mated fire ant queens. Myriad interactions occur within founding colonies, including cannibalism of both queens and larvae. After the claustral period, brood raiding (brood stealing contests among newly-founded neighboring colonies) under natural conditions shapes the population dynamics of colony founding and early colony growth.

 (5) Food traffic. Among ants, only the oldest workers leave the nest to forage. They bring liquid food back to the nest in their crops and share it with nestmates through a complex web of regurgitation. We illuminated the quantitative movement of food within the colony, showed that the global patterns result from local decisions based on local information, and discovered the “rules” by which food is shared with larvae and other workers. This is also the first detailed study of the involvement of larvae in the colony’s food web.
(5) Food traffic. Among ants, only the oldest workers leave the nest to forage. They bring liquid food back to the nest in their crops and share it with nestmates through a complex web of regurgitation. We illuminated the quantitative movement of food within the colony, showed that the global patterns result from local decisions based on local information, and discovered the “rules” by which food is shared with larvae and other workers. This is also the first detailed study of the involvement of larvae in the colony’s food web. (6) Evolution of colony life histories: The methods of sociometry/ sociogenesis, provide a simple procedure for the simultaneous determination of suites of colony characteristics and how they develop during colony growth. This is the social insect counterpart of embryonic development, and has received little attention. Sociometric/sociogenic studies on fire ants and harvester ants produced the first quantitative, full life-history descriptions of an ant colony, from birth to death.
(6) Evolution of colony life histories: The methods of sociometry/ sociogenesis, provide a simple procedure for the simultaneous determination of suites of colony characteristics and how they develop during colony growth. This is the social insect counterpart of embryonic development, and has received little attention. Sociometric/sociogenic studies on fire ants and harvester ants produced the first quantitative, full life-history descriptions of an ant colony, from birth to death.  (7) Worker polymorphism. Workers in fire ant colonies vary about 15-fold in weight from the smallest to the largest. This so-called polymorphism develops gradually as colonies grow. Detailed descriptions of polymorphism of colonies of a full range of sizes reveals how polymorphism arises during colony development. Polymorphism has been hypothesized to make colonies more efficient and therefore to increase their fitness. We tested this in experiments that used brood rearing efficiency as a surrogate of fitness.
(7) Worker polymorphism. Workers in fire ant colonies vary about 15-fold in weight from the smallest to the largest. This so-called polymorphism develops gradually as colonies grow. Detailed descriptions of polymorphism of colonies of a full range of sizes reveals how polymorphism arises during colony development. Polymorphism has been hypothesized to make colonies more efficient and therefore to increase their fitness. We tested this in experiments that used brood rearing efficiency as a surrogate of fitness.
 (9) Fire ant, native ant interactions. The fire ant is an exotic invasive ant from Brazil that is of great political and economic importance. Ecological work on this species has clearly demonstrated that ecological disturbance favors the establishment and existence of this “weedy” species, making humans the fire ant’s best friend. Moreover, the long-held belief that fire ants suppress native ant populations was shown to be false.
(9) Fire ant, native ant interactions. The fire ant is an exotic invasive ant from Brazil that is of great political and economic importance. Ecological work on this species has clearly demonstrated that ecological disturbance favors the establishment and existence of this “weedy” species, making humans the fire ant’s best friend. Moreover, the long-held belief that fire ants suppress native ant populations was shown to be false.  (10) Other fire ant biology. In spite of the importance of fire ants, much of their biology is unknown or poorly understood. A number of our papers illuminate important aspects of the biology of this ant, including its capacity to regulate larval development by choosing developmental temperatures, the longevity of its queens and colonies, its sanitation behavior, its brood-care signals, sperm storage, food storage, organization of foraging and the effect of temperature on foraging.
(10) Other fire ant biology. In spite of the importance of fire ants, much of their biology is unknown or poorly understood. A number of our papers illuminate important aspects of the biology of this ant, including its capacity to regulate larval development by choosing developmental temperatures, the longevity of its queens and colonies, its sanitation behavior, its brood-care signals, sperm storage, food storage, organization of foraging and the effect of temperature on foraging. (11) Architecture of subterranean ant nests. We have pioneered the study of nest architecture of ground-nesting ants through the creation of nest casts using orthodontal plaster or liquid metal. This work is still in progress, but has received national and international attention. The initial goal of this research is to provide an inventory of the structures ants create, as well as an understanding of how colonies are organized to produce these structures.
(11) Architecture of subterranean ant nests. We have pioneered the study of nest architecture of ground-nesting ants through the creation of nest casts using orthodontal plaster or liquid metal. This work is still in progress, but has received national and international attention. The initial goal of this research is to provide an inventory of the structures ants create, as well as an understanding of how colonies are organized to produce these structures. (12) Natural history of arboreal ants. With the discovery by Dr. Frances James and Charles Hess that the endangered red-cockaded woodpecker ate mostly a single species of tree-dwelling ant, Crematogaster ashmeadi, it became important to learn as much as possible about the ecology and natural history of this critical ant. Subsequently, we have worked out most of the basic outlines of the colony cycle, natural history, distribution and abundance of this ant. In the process, we discovered that this previously unknown ant is the most abundant and dominant arboreal ant in the coastal plain pine forests of the southern states.
(12) Natural history of arboreal ants. With the discovery by Dr. Frances James and Charles Hess that the endangered red-cockaded woodpecker ate mostly a single species of tree-dwelling ant, Crematogaster ashmeadi, it became important to learn as much as possible about the ecology and natural history of this critical ant. Subsequently, we have worked out most of the basic outlines of the colony cycle, natural history, distribution and abundance of this ant. In the process, we discovered that this previously unknown ant is the most abundant and dominant arboreal ant in the coastal plain pine forests of the southern states.  (13) Natural history of other ants. The natural history of most ant species is, at best, poorly known. Adding to the store of natural history knowledge allows inferences about the evolution of ant sociality, ecology and behavior to be drawn. A number of “background” projects on the natural history of local species of ants have revealed novel and fascinating things about the lives of Florida’s ants.
(13) Natural history of other ants. The natural history of most ant species is, at best, poorly known. Adding to the store of natural history knowledge allows inferences about the evolution of ant sociality, ecology and behavior to be drawn. A number of “background” projects on the natural history of local species of ants have revealed novel and fascinating things about the lives of Florida’s ants.  (14) Harvester ants and fungus gardening ants. We have studied the natural history of some local ants more intensively than others. Two in particular stand out--- the Florida harvester ant, and the fungus gardening ant, Trachymyrmex septentrionalis. Each species does interesting things that invite explanation. The harvester ant collects seeds for food, but also collects charcoal pieces to decorate its nest (the detailed sociometry of this species was noted in item 5, above). The fungus-gardening ant collects plant bits and caterpillar droppings for its fungus gardens, raising the question, “does it know what is good for its fungus?”
(14) Harvester ants and fungus gardening ants. We have studied the natural history of some local ants more intensively than others. Two in particular stand out--- the Florida harvester ant, and the fungus gardening ant, Trachymyrmex septentrionalis. Each species does interesting things that invite explanation. The harvester ant collects seeds for food, but also collects charcoal pieces to decorate its nest (the detailed sociometry of this species was noted in item 5, above). The fungus-gardening ant collects plant bits and caterpillar droppings for its fungus gardens, raising the question, “does it know what is good for its fungus?” (15) Chemical defense, pheromones and systematics of tenebrionid and other beetles. Tenebrionid beetles are a cosmopolitan family of about 20,000 species, most of whom possess a chemical defensive system. We carried out the largest and most complete comparative study of this chemical defense system, including analysis of the chemical composition of the secretion, anatomy of the glands, and defensive behavior. This remains a standard-setting study that has rarely been equaled or surpassed. These comparative studies culminated in the revision of the higher systematics of family Tenebrionidae.
(15) Chemical defense, pheromones and systematics of tenebrionid and other beetles. Tenebrionid beetles are a cosmopolitan family of about 20,000 species, most of whom possess a chemical defensive system. We carried out the largest and most complete comparative study of this chemical defense system, including analysis of the chemical composition of the secretion, anatomy of the glands, and defensive behavior. This remains a standard-setting study that has rarely been equaled or surpassed. These comparative studies culminated in the revision of the higher systematics of family Tenebrionidae.  (16) Inhibition of metamorphosis by crowding. Crowding in tenebrionid beetle larvae has a novel effect in that it directly inhibits metamorphosis through the effects of larval-larval contacts. We worked out its natural history, and through experimentation, described the mechanism of the inhibition and its life-history consequences in Zophobas atratus. WE also demonstrated the importance of this effect in natural populations.
(16) Inhibition of metamorphosis by crowding. Crowding in tenebrionid beetle larvae has a novel effect in that it directly inhibits metamorphosis through the effects of larval-larval contacts. We worked out its natural history, and through experimentation, described the mechanism of the inhibition and its life-history consequences in Zophobas atratus. WE also demonstrated the importance of this effect in natural populations. 
